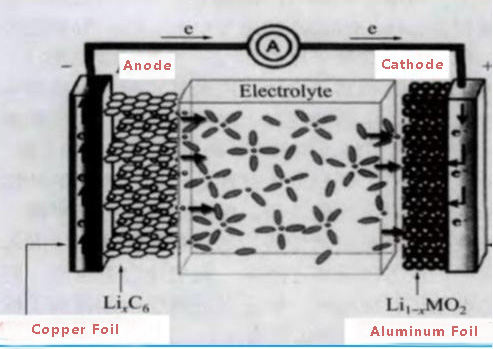
For lithium-ion batteries, the commonly used positive current collector is aluminum foil, and the negative electrode current collector is copper foil. In order to ensure the stability of the current collector inside the battery, the purity of both is required to be above 98%. The working principle of lithium batteries is an electrochemical device that converts chemical energy into electrical energy. In this process, we need a medium to transfer the electrical energy converted from chemical energy, and conductive materials are needed here. Among ordinary materials, metal materials are the most conductive materials, and among metal materials, copper foil and aluminium foil are cheap and have good conductivity.

There are 6 reasons why the positive electrode of lithium-ion batteries likes to use aluminum foil:
1. Aluminum Foil is Relatively Stable in the Air
Aluminum easily reacts with oxygen in the air, forming a dense oxide film on the surface of the aluminum to prevent further reaction of aluminium, and this thin oxide film also has a certain protective effect on aluminum in the electrolyte.
2. The Copper Foil is Easily Oxidized at High Potential
The potential of the positive and negative electrodes of the lithium battery determines the aluminum foil for the positive electrode.
The positive electrode potential is high, and the copper foil is easily oxidized at high potential, while the oxidation potential of aluminum is high, and the surface of the aluminum foil has a dense oxide film, which also has a good protective effect on the internal aluminium. Both of them are used as current collectors because they have good electrical conductivity, soft texture (perhaps this will also be conducive to bonding), and are relatively common and cheap. At the same time, a layer of oxide protective film can be formed on the surface of both.

3. High Activity of the Reaction Between Metal Al and Li
The lattice octahedral void size of metal aluminum is similar to that of Li, and it is easy to form interstitial compounds with Li. Li and Al not only form an alloy with the chemical formula Li-Al, but also Li3Al2 or Li4Al3. Due to the high activity of the reaction between metal Al and Li, the metal Al consumes a large amount of Li, and its structure and shape are also damaged, so it cannot be used as the current collector of the negative electrode of lithium-ion batteries; It has only a small amount of lithium intercalation capacity, and maintains the stable structure and electrochemical performance, and can be used as a current collector for the negative electrode of lithium-ion batteries; when the Cu foil is at 3.75V, the polarization current begins to increase significantly, and it rises linearly, oxidation Intensified, indicating that Cu is unstable at this potential; while the polarization current of aluminium foil is small and constant in the entire polarization potential range, no obvious corrosion phenomenon is observed, and the electrochemical performance is stable. Due to the small lithium intercalation capacity of Al in the positive electrode potential range of lithium-ion batteries, and can maintain electrochemical stability, it is suitable as a positive electrode current collector for lithium-ion batteries.
4. The Oxide Layer Cannot Conduct Electricity
The oxide layer on the copper/nickel surface is a semiconductor, and the electrons are conductive, the oxide layer is too thick, and the impedance is large; while the oxide layer on the aluminum surface is an aluminum oxide insulator, and the oxide layer cannot conduct electricity, but due to its thinness, electrons are realized through the tunnel effect. Conductivity, if the oxide layer is thick, the conductivity of the aluminium foil is poor, or even insulation. Generally, the current collector should be cleaned on the surface before use. On the one hand, the oil stains can be washed off, and the thick oxide layer can be removed at the same time.
5. The Aluminum Foil Oxide Layer Prevents the Current Collector from Oxidizing
The positive electrode potential is high, and the thin aluminum oxide layer is very dense, which prevents the current collector from oxidizing. However, the oxide layer of copper/nickel foil is looser. In order to prevent its oxidation, it is better to have a lower potential. At the same time, it is difficult for Li to form a lithium intercalation alloy with Cu/nickel at a low potential. Li reacts with copper oxide/nickel under potential. While aluminium foil cannot be used as a negative electrode, Li-Al alloying occurs at low potential.
6. The Current Collector Requires Pure Composition.
The impure composition of Al will lead to the occurrence of pitting corrosion because the surface film is not dense, and even the formation of Li-Al alloy due to the damage of the surface film.